Calcium ions play a vital role in biological systems, contributing to enzyme activity, cellular signalling pathways, muscle contraction, and neuronal functioning. Calcium assays rely on dye-binding probes, which change colour or emit fluorescence when present.
In this blog, we look deeper into the technical aspects of calcium assay methods, discuss their significance in various fields such as biochemistry, cell biology, and pharmacology, and examine case studies where calcium assays have played a pivotal role.
Whether you’re a seasoned researcher, a student embarking on your scientific journey, or simply a science enthusiast, this blog aims to enlighten you about the fascinating role of calcium in biological systems and the innovative techniques used to study it. Join us as we unravel the complexities of calcium signalling pathways, their impact on cellular functions, and the latest advancements in calcium assay technologies. Our expert contributors and engaging content make “Calcium Assays and Their Uses” your go-to resource for everything related to this essential field of study. Welcome aboard!
Calcium Flux Assay
Calcium is essential for both cellular and physiological functions, including enzyme activity, muscle contraction, neuronal signalling, hormone balance, and more. Calcium exists primarily in two forms in the body: total calcium and ionised calcium. Total calcium is usually measured through blood tests, while ionised calcium measures its free form, which is metabolically active; in many instances, this form may provide more accurate measures of drug effects on body systems.
Agonists or antagonists activate G protein-coupled receptors (GPCRs) by using intracellular calcium levels as a signal. These assays utilise fluorescent dye or luminescent aequorin calcium indicators to monitor calcium mobilisation within cells and the release of Ca2+ into the cytoplasm. These techniques are widely used during high-throughput screening campaigns and serve as invaluable tools for hit identification, characterization, and confirmation in GPCR-based target discovery.
Fluorescent calcium indicators are often used by cells, which preload them with a fluorescent dye that binds to calcium released from within cells and increases fluorescence intensity upon stimulation of GPCRs. When a GPCR is activated, the cell’s storage pools let calcium into the cytoplasm. The fluorescence intensity directly shows the amount of calcium inside the cell.
Using luminescent markers of aequorin in assays makes it possible to find changes in Ca2+ homeostasis without using radioactive markers. This makes the assays very useful for finding new channels and makes them easy to scale up for high-throughput screening.
Molecular Probes has created ion indicators that can give sensitive, accurate, and repeatable readings similar to patch clamp assays but in an automated format that can be used for high-throughput screening. Our aequorin-based calcium indicators have better signal stability, less background interference during readout, and more than one wavelength to choose from to make the assay design as efficient as possible.
G Protein-Coupled Receptor (GPCR) Activation-Evoked Increases in Intracellular Ca2+
G protein-coupled receptor (GPCR) activator-evoked increases in intracellular calcium are an established measure of GPCR signalling, making cell-based calcium mobilisation assays widely utilised to quickly assess GPCR activation by drugs or therapeutic agents in a high-throughput manner.
As soon as G protein-coupled adenylyl cyclase (GPCR) proteins were discovered and cloned in the early 1990s, they ignited renewed interest in calcium signalling [2]. GPCRs are protein ligands that are known to quickly switch on and off cytoplasmic calcium channels when they sense an agonist. This causes a sudden Ca2+ transient inside cells.
Fluorescent readouts can measure recombinant GPCRs that are expressed in cells along with a GPCR-specific Ca2+ ion indicator to see if the GPCRs cause calcium levels to rise inside cells. Aequorin, a jellyfish-derived photoprotein biosensor used as an indicator, detects changes in calcium concentration by binding with calcium molecules, converting into coelenteramide, and emitting luminescent signals. This technique is highly sensitive yet simple for microplate readers such as the Centro or Tristar luminometer.
Alternative cell-based calcium assays using polycyclic chelators such as Indo-1 or Fura-2 provide ratio metric readouts less prone to diffusion signal loss from the cell membrane, providing physiologically relevant measures of GPCR signalling than Schild analysis despite taking more time to incubate for a complete Ca2+ transient.
Eurofins DiscoverX offers stable GPCR cell lines, ready-to-test frozen cells, and the Calcium No WashPLUS detection kit so that you can quickly and easily study how GPCR agonists affect calcium mobilisation.
Cell-Based Calcium Mobilisation Assays
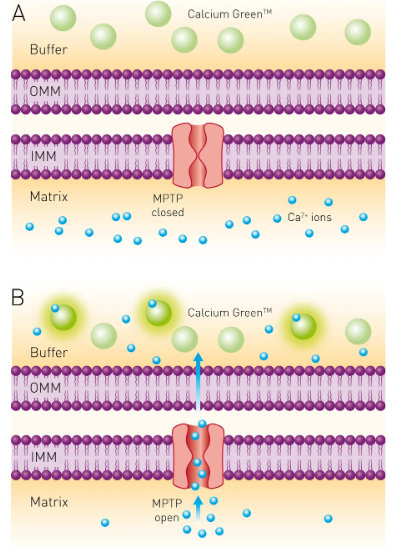
Calcium (Ca2+) plays an essential role in many aspects of cell function, from enzyme activity and signal transduction through muscle contraction to neuronal signaling. Many different assays exist for measuring Ca2+ levels in cells or biological samples using chemical or biophysical methods; one such assay involves adding a Ca2+ indicator that changes colour or generates fluorescence depending on how much Ca2+ is present. These markers can be useful for tracking cellular and physiological processes as well as hormone imbalances or drug effects.
Calcium flux assays provide an efficient means of studying how neurons derived from human-induced pluripotent stem cells (iPSC) operate. For the study below, a FLIPR calcium-6 assay kit and FlexStation 3 Multi-Mode Microplate Reader were utilised to monitor iPSC-derived neurons and quantify variations in Ca2+ concentrations associated with neuronal function. Peak Pro Analysis software enabled the quantification and calculation of important peak attributes like peak count, width, height, frequency, etc.
Synthetic calcium indicators
Researchers have created multiple chemical calcium indicators and sensors to enhance the accuracy and sensitivity of cellular calcium measurements. Many are based on commonly-used calcium chelators such as EGTA (ethylene glycol-bis(b-aminoethyl ether)) or more sophisticated ones with improved calcium ion specificity, pH stability, and Ca2+ affinity, such as BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N’,N’tetraacetic acid). Calcium binding to these indicator molecules results in changes to their spectral properties that can be detected using fluorescent microscopy.
Common indicators typically demonstrate either an increase in quantum yield or shifts in emission or excitation wavelength; for instance, the popular green fluorophore GCaMP6s shows strong red-shifted fluorescence when bound with calcium; other indicators, like the highly selective calcium sensor QCaMP-2, show a marked change in emission maximum wavelength at 525nm when Ca2+ binds, making them suitable for applications like confocal laser scanning microscopy.
Recently, genetically encoded calcium indicators (GECIs) have become an invaluable asset to assay workflows and increasing precision. These GECIs can be expressed stably in mammalian cells to detect calcium flux events without needing cell lysis or preparation for analysis.
GECIs are great for keeping track of how drugs change the concentration and flow of Ca2+ because they give accurate readings of both the rate of influx and accumulation. This is important because a faster Ca2+ transient, with its activation of downstream signalling pathways, may provide a more relevant indication of pharmacological activity than the slower Ca2+ influx and decay seen with synthetic indicators.
Genetically encoded calcium indicators
Genetically encoded calcium indicators (GECIs) have proven an invaluable asset in imaging calcium signaling. Utilising fluorescent proteins as reporters, GECIs have become one of the primary approaches for understanding calcium signalling (17). One such GECI was aequorin, purified from Aequorea Victoria jellyfish and introduced into cells during experiments conducted in the 1970s (17). Aequorin required coelenterazine as a substrate to produce its luminescent signal; when calcium-bound, this caused coelenterazine to oxidise, further producing fluorescent light that could be detected using charge-coupled devices (CCDs).
GECIs may also be combined with green fluorescent protein (GFP) or its variants eGFP, YFP, and CFP in order to simultaneously report on both Ca2+ levels and other biological events such as cell target phosphorylation and transport of vesicles. Researchers can direct GECIs towards subcellular organelles like mitochondria.
Most GECIs operate via fluorescence resonance energy transfer (FRET). Calcium binding to an indicator protein causes conformational changes that cause FRET, leading to an increase in fluorescence. There are multiple available GECIs with red-shifted emission spectra that allow measurements in deep tissues.
Calcium indicators also include small molecules that chelate calcium ions with high selectivity for calcium over magnesium (Mg2+). Chemical indicators include EGTA homologues like fura-2, indo-1, and fluo-3, as well as the more powerful emission-shifted fluo-4; all can be used to monitor cell-cytosolic calcium concentration, typically 10-100 nM at rest but able to increase by 100x+ upon stimulation; they’re widely employed in GPCR receptor pharmacology research studies.

Rachel is a print media specialist with expertise in traditional and digital printing techniques, exploring their impact on branding and marketing.

